Overview of Lithium-Ion Batteries
Exploring the Science, Technology, and Applications of Modern Energy Storage
Lithium-ion batteries (LIBs) have become the cornerstone of modern energy storage solutions, powering everything from portable electronics to electric vehicles and grid-scale energy storage systems. What are lithium batteries? They are rechargeable energy storage devices that utilize lithium ions as the key component of their electrochemistry. This article delves into the intricate details of lithium-ion batteries, exploring their electrochemical principles, raw materials, manufacturing processes, performance characteristics, and diverse applications.
Electrochemical Principles of Lithium-Ion Batteries
At the heart of every lithium battery lies a sophisticated electrochemical system that enables the storage and release of energy through the movement of lithium ions between the anode and cathode. What are lithium batteries in terms of electrochemistry? They are devices that rely on redox reactions to store and release electrical energy.
Basic Electrochemical Reactions
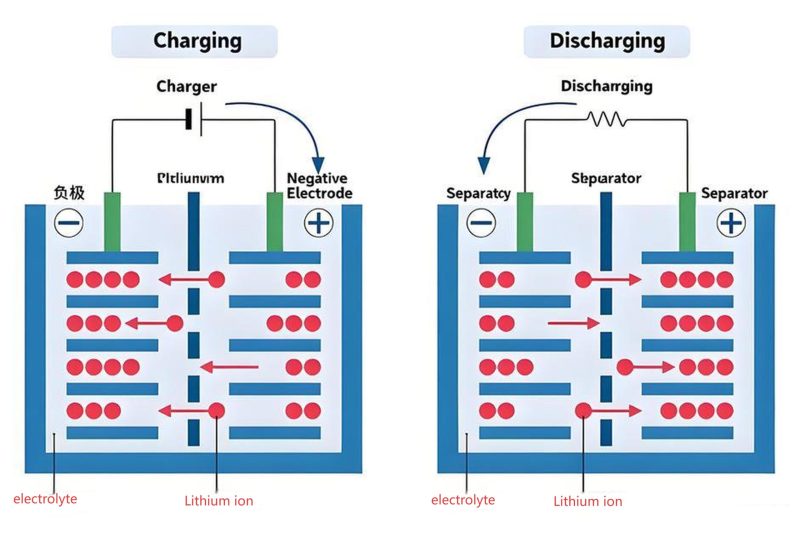
During charging, lithium ions are extracted from the cathode (positive electrode) and inserted into the anode (negative electrode) through an electrolyte, while electrons flow through an external circuit. The process is reversed during discharging, with lithium ions moving back to the cathode and electrons providing electrical current.
Charging Reaction
Cathode: LiCoO₂ → Li₁₋ₓCoO₂ + xLi⁺ + xe⁻
Anode: xLi⁺ + xe⁻ + 6C → LiₓC₆
Discharging Reaction
Anode: LiₓC₆ → xLi⁺ + xe⁻ + 6C
Cathode: Li₁₋ₓCoO₂ + xLi⁺ + xe⁻ → LiCoO₂
Working Mechanism
Lithium-ion batteries operate based on the "rocking-chair" principle, where lithium ions move back and forth between the anode and cathode during charge and discharge cycles. This movement is facilitated by the electrolyte, a conductive medium that allows ion transport while preventing electron flow between the electrodes.
Electrode Materials
The choice of electrode materials significantly impacts the performance of lithium-ion batteries. Common cathode materials include lithium cobalt oxide (LiCoO₂), lithium manganese oxide (LiMn₂O₄), lithium iron phosphate (LiFePO₄), and lithium nickel manganese cobalt oxide (NMC). Graphite is the most widely used anode material due to its ability to intercalate lithium ions effectively.
Cell Potential
The cell potential of a lithium-ion battery is determined by the difference in electrochemical potential between the cathode and anode materials. For example, a typical LiCoO₂/graphite cell has a nominal voltage of 3.7V, significantly higher than other rechargeable battery systems such as lead-acid (2V) or nickel-metal hydride (1.2V).
Lithium-Ion Battery Electrochemical Cell
Schematic representation of a typical lithium-ion battery cell during discharge. Lithium ions (Li⁺) move from the anode to the cathode through the electrolyte, while electrons (e⁻) flow through the external circuit, providing electrical energy.
Key Concept
The efficiency of lithium-ion batteries stems from their reversible electrochemical reactions, which allow for repeated charging and discharging cycles with minimal degradation.
FAQ
What are lithium batteries' energy density compared to other types? Lithium-ion batteries typically offer 100-265 Wh/kg, significantly higher than lead-acid (30-50 Wh/kg) or NiMH (60-120 Wh/kg) batteries.
Raw Materials and Manufacturing of Lithium-Ion Batteries
The production of high-performance lithium-ion batteries relies on specialized raw materials and manufacturing processes that ensure consistency, safety, and efficiency.
what is in a lithium ion battery Key Raw Materials
Lithium Sources
Lithium is primarily extracted from brine deposits (e.g., Salar de Atacama in Chile) and hard rock minerals such as spodumene. The most common lithium compounds used in batteries include lithium carbonate (Li₂CO₃) and lithium hydroxide (LiOH).
Cathode Materials
Cathode materials require transition metals such as cobalt, nickel, manganese, and iron. Cobalt is critical for enhancing battery stability and energy density, while nickel increases capacity. However, the high cost and ethical concerns associated with cobalt mining have spurred research into low-cobalt and cobalt-free alternatives.
Anode Materials
Graphite is the dominant anode material due to its layered structure, which allows for efficient intercalation of lithium ions. Emerging anode materials include silicon, which offers much higher theoretical capacity than graphite but faces challenges related to volume expansion during charging.
Electrolytes and Separators
The electrolyte is typically a lithium salt (e.g., LiPF₆) dissolved in an organic solvent. Separators, usually made of porous polyolefin membranes, physically separate the anode and cathode while allowing lithium ion transport.

Lithium Extraction
Lithium brine pools in Chile's Atacama Desert. The region accounts for nearly 40% of global lithium production, highlighting the geographical concentration of key battery raw materials.
Manufacturing Process
The manufacturing of lithium-ion batteries involves multiple complex steps that require precision control to ensure battery performance and safety.
Electrode Slurry Preparation
Active materials (cathode and anode), conductive additives, and binders are mixed with solvents to form homogeneous slurries. These slurries are then coated onto metallic foils (aluminum for cathodes, copper for anodes) using precision coating techniques.
Electrode Drying and Calendaring
The coated foils are dried to remove solvents, and then calendared to compress the electrode materials, improving their density and electrical conductivity. This step is critical for achieving high energy density and uniform performance.
Cell Assembly
The electrodes are cut to size and assembled with separators between them. This assembly is then wound or stacked into a cell casing, which is typically made of aluminum or steel for prismatic cells or aluminum laminate for pouch cells.
Electrolyte Filling and Sealing
The assembled cell is filled with electrolyte under controlled humidity conditions to prevent contamination. The cell is then hermetically sealed to prevent electrolyte leakage and ingress of moisture.
Formation and Aging
The cells undergo an initial charge-discharge cycle (formation) to create a solid electrolyte interphase (SEI) on the anode surface, which is critical for battery stability and cycle life. The cells are then aged to ensure performance consistency before final testing.
Testing and Quality Control
Final cells are tested for capacity, voltage, internal resistance, and self-discharge rate. Cells that meet specifications are grouped into modules and packs, which include battery management systems (BMS) to monitor and control performance.
Environmental Considerations
Lithium extraction and battery manufacturing have significant environmental impacts, including water usage, energy consumption, and greenhouse gas emissions. Sustainable practices and recycling initiatives are critical for reducing the industry's environmental footprint.
Supply Chain Challenges
The global supply chain for lithium-ion battery materials is complex and faces challenges such as price volatility, geopolitical risks, and ethical concerns related to mining practices. Diversification of sourcing and development of alternative materials are key strategies to address these issues.
Technological Innovations
Research is ongoing to develop new materials and manufacturing processes that reduce costs, improve performance, and enhance sustainability. Solid-state electrolytes, silicon anodes, and lithium-sulfur chemistries are among the most promising emerging technologies.

Energy Density Range
Typical values for commercial lithium-ion batteries, depending on chemistry and design.
Charge Efficiency
High efficiency reduces energy loss during charging cycles.
Cycle Life
Number of charge-discharge cycles before reaching 80% of original capacity.
Voltage Range
Typical voltage window for most lithium-ion battery chemistries.
Performance Characteristics of Lithium-Ion Batteries
Ithium Ion Battery Energy Density.The performance of lithium-ion batteries is determined by a combination of electrochemical properties, material characteristics, and design parameters. What are lithium batteries' key performance metrics? They include energy density, power density, cycle life, charging efficiency, and safety.
Energy and Power Density
Energy density (Wh/kg) refers to the amount of energy stored per unit mass, while power density (W/kg) measures the rate at which energy can be delivered. High energy density is critical for applications requiring long runtime, such as electric vehicles, while high power density is essential for devices needing rapid energy delivery, like power tools.
Cycle Life and Calendar Life
Cycle life refers to the number of complete charge-discharge cycles a battery can undergo before its capacity drops to a specified percentage (typically 80%) of its original capacity. Calendar life, on the other hand, is the amount of time a battery can remain functional regardless of usage.
Factors Affecting Cycle Life
- Depth of discharge (DOD)
- Charge and discharge rates
- Operating temperature
- Battery chemistry
Extending Battery Life
- Avoid deep discharges (keep DOD below 80%)
- Charge at moderate rates
- Store batteries at 40-60% state of charge
- Maintain optimal operating temperatures
Safety and Thermal Management
Safety is a critical performance aspect of lithium-ion batteries. While generally safe, they can pose risks such as thermal runaway if overheated, overcharged, or physically damaged. Advanced battery management systems (BMS) and thermal management systems (TMS) are employed to monitor and control battery conditions.
Safety Features in Modern Batteries
Overcharge Protection
BMS prevents charging beyond safe voltage limits.
Thermal Sensors
Monitor temperature to prevent overheating.
Current Limiting
Prevents excessive current flow during charging/discharging.
Pressure Relief Valves
Release gas in case of overpressure to prevent explosions.
Development, Characteristics, and Applications of Lithium-Ion Batteries
From their humble beginnings to powering the modern world, lithium-ion batteries have revolutionized energy storage across diverse industries.
Development and Historical Context
Early Research
The concept of lithium-based batteries dates back to the 1970s, with initial research focusing on non-rechargeable lithium batteries. The development of rechargeable lithium-ion batteries began in the 1980s, with significant contributions from John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino, who shared the 2019 Nobel Prize in Chemistry for their work.Lithium ion battery.
Commercialization
Sony commercialized the first lithium-ion battery in 1991, marking a major milestone in portable electronics. The technology quickly replaced nickel-cadmium (NiCd) batteries due to its superior energy density, lack of memory effect, and longer cycle life.
Recent Advancements
Ongoing research has led to the development of new cathode materials (such as NMC and LFP), solid-state electrolytes, and silicon-based anodes, pushing the boundaries of energy density, safety, and cost-effectiveness.
Timeline of Key Developments
M. Stanley Whittingham develops the first rechargeable lithium battery using titanium disulfide as cathode and lithium metal as anode.
John B. Goodenough discovers lithium cobalt oxide (LiCoO₂) cathode, doubling the voltage of lithium batteries.
Akira Yoshino develops the first commercially viable lithium-ion battery using a carbon-based anode, solving the safety issues associated with lithium metal anodes.
Sony commercializes the first lithium-ion battery for portable electronics.
Lithium iron phosphate (LiFePO₄) cathode material is developed, offering improved safety and lower cost.
Lithium nickel manganese cobalt oxide (NMC) cathodes are introduced, becoming the dominant chemistry for electric vehicles.
Large-scale deployment of lithium-ion batteries in electric vehicles and grid energy storage systems.
Research focuses on solid-state batteries, silicon anodes, and sustainable battery materials.
Key Characteristics
High Energy Density
Lithium-ion batteries offer the highest energy density among commercial rechargeable batteries, making them ideal for portable devices and electric vehicles.
Low Self-Discharge
Lithium-ion batteries have a low self-discharge rate of about 1-2% per month, compared to 20-30% for NiMH batteries.
No Memory Effect
Unlike NiCd batteries, lithium-ion batteries do not suffer from memory effect, allowing them to be recharged at any state of charge without reducing capacity.
High Voltage
Single lithium-ion cells typically have a nominal voltage of 3.6-3.7V, compared to 1.2V for NiMH and NiCd batteries, reducing the number of cells required in series.
Long Cycle Life
Modern lithium-ion batteries can last for 500-2000+ charge-discharge cycles, depending on the chemistry and usage conditions.
Environmentally Friendly
Lithium-ion batteries contain fewer toxic materials compared to lead-acid or NiCd batteries, and recycling programs are becoming more widespread.
Applications

Portable Electronics
Lithium-ion batteries are the primary power source for smartphones, laptops, tablets, smartwatches, and wireless earbuds due to their high energy density and compact size.
- Smartphones and tablets
- Laptops and notebooks
- Wearable devices
- Portable power tools
Electric Vehicles (EVs)
The automotive industry relies heavily on lithium-ion batteries to power electric cars, buses, trucks, and e-bikes, enabling longer driving ranges and faster charging times.
- Battery electric vehicles (BEVs)
- Plug-in hybrid electric vehicles (PHEVs)
- Electric buses and trucks
- E-bikes and electric scooters

Grid Energy Storage
Large-scale lithium-ion battery systems are used for grid stabilization, storing excess energy from renewable sources like solar and wind, and providing backup power during outages.
- Peak shaving and load balancing
- Integration of renewable energy
- Backup power for critical facilities
- Microgrid applications

Renewable Energy Storage
Lithium-ion batteries play a crucial role in storing energy generated from solar panels and wind turbines, making renewable energy sources more reliable and accessible.
- Residential solar storage systems
- Commercial and industrial energy storage
- Off-grid power systems
- Hybrid renewable energy systems

Medical Devices
Critical medical equipment such as portable monitors, defibrillators, and insulin pumps rely on lithium-ion batteries for their reliability, long life, and high energy density.
- Portable medical monitors
- Implantable medical devices
- Defibrillators and emergency equipment
- Insulin pumps and drug delivery systems

Aerospace and Military
In aerospace and military applications, lithium-ion batteries are used for their high energy-to-weight ratio, reliability, and ability to operate in extreme environments.Electronic shelf labels.
- Unmanned aerial vehicles (UAVs)
- Satellites and space probes
- Military portable equipment
- Electric propulsion systems
Conclusion
Lithium-ion batteries have revolutionized the way we store and use energy, powering everything from our smartphones to electric vehicles and grid-scale energy storage systems. Their high energy density, long cycle life, and low self-discharge rate make them the preferred choice for a wide range of applications. As research continues to advance, future developments in battery technology, such as solid-state electrolytes and sustainable materials, promise to further enhance performance, safety, and sustainability.Related Hydraulic Spare Parts.
More Related