LIBTechInsight
Principle and Application of Lithium Ion Battery Manufacturing Technology
Explore the comprehensive guide to lithium ion battery manufacturing, from raw materials to advanced applications.

3,000+ Words
In-Depth Guide
Lithium Ion Battery Overview
What are lithium batteries?Lithium ion batteries (LIBs) have become the dominant energy storage solution for portable electronics and electric vehicles due to their high energy density, long cycle life, and low self-discharge rate. The first commercial lithium ion battery was introduced by Sony in 1991, revolutionizing the consumer electronics industry.
The basic structure of a lithium ion battery consists of a cathode (positive electrode), an anode (negative electrode), a separator, and an electrolyte. During charging, lithium ions move from the cathode to the anode through the electrolyte, while electrons flow through the external circuit. During discharge, the reverse process occurs.
Key Characteristics of LIBs
- High energy density: 100-265 Wh/kg
- Low self-discharge rate: ~5% per month
- Long cycle life: 500-1000 cycles
- No memory effect
The success of lithium ion batteries can be attributed to their unique electrochemical properties and the continuous research and development efforts to improve their performance, safety, and cost-effectiveness.
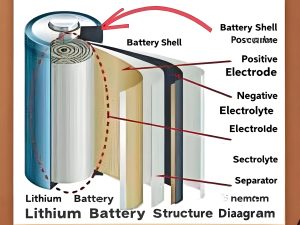
Figure 1: Basic Structure of a Lithium Ion Battery
High Energy Density
Rechargeable
Lithium Ion Battery Raw Materials
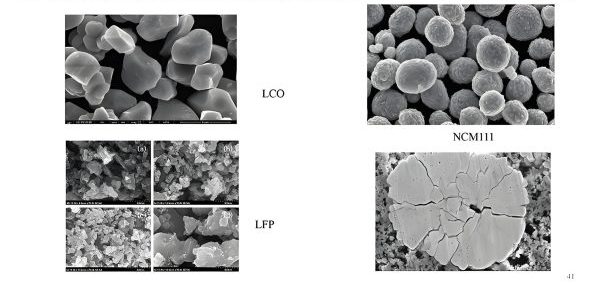
Figure 2: Key Raw Materials for LIB Production
The performance and cost of lithium ion batteries are heavily influenced by the choice of raw materials. The main components include cathode materials, anode materials, electrolytes, separators, and current collectors.lithium ion battery fire.
Cathode Materials
Cathode materials are critical as they determine the energy density, voltage, and cost of the battery. Common cathode materials include:
Lithium Cobalt Oxide (LCO)
High energy density, used in smartphones and laptops.
Lithium Manganese Oxide (LMO)
Low cost, good thermal stability, used in power tools.
Lithium Iron Phosphate (LFP)
Long cycle life, high safety, used in electric vehicles.
Nickel Cobalt Aluminum Oxide (NCA)
High energy density, used in Tesla vehicles.
Anode Materials
The most common anode material is graphite due to its low cost, good cycling performance, and low lithium insertion potential. Other materials include lithium titanate (LTO) and silicon-based anodes.
Silicon Anodes
Silicon has attracted significant attention due to its high theoretical capacity (4200 mAh/g), which is more than 10 times higher than graphite (372 mAh/g). However, silicon anodes face challenges related to volume expansion during charging and discharging.
Electrolytes and Separators
The electrolyte is typically a lithium salt dissolved in an organic solvent, while the separator is a porous membrane that prevents short circuits while allowing lithium ions to pass through.
Lithium Ion Battery Porous Electrode Basics
Porous electrodes play a crucial role in lithium ion batteries as they provide a large surface area for electrochemical reactions, facilitate ion and electron transport, and affect the overall performance of the battery.Lithium ion battery aa battery.
Electrode Structure and Performance
A typical porous electrode consists of active material particles, conductive additives (such as carbon black), and a binder (such as PVDF) on a current collector. The structure of the electrode affects:
- Ion diffusion: Pore size distribution and tortuosity affect lithium ion diffusion rates.
- Electron conduction: Conductive additive network influences electron transport.
- Reaction kinetics: Surface area and porosity affect the rate of electrochemical reactions.
Optimizing the electrode structure is critical for achieving high energy density, power density, and long cycle life in lithium ion batteries.
Electrode Manufacturing Considerations
The manufacturing process of porous electrodes involves several key steps that influence the final electrode structure:
Particle Size Distribution
Optimizing particle size can improve packing density and reduce tortuosity.
Binder Content
Higher binder content improves mechanical stability but reduces conductivity.
Coating Thickness
Thicker coatings increase energy density but may reduce power performance.
Drying Process
Proper drying is critical to prevent cracking and ensure uniform structure.
Modeling and Characterization
Advanced modeling and characterization techniques are used to understand and optimize porous electrode structures:
Electrochemical Impedance Spectroscopy (EIS)
Measures ion and electron transport properties.
Scanning Electron Microscopy (SEM)
Imaging of electrode microstructure.
Computational Modeling
Predicts performance based on structural parameters.
These techniques help researchers and engineers design electrodes with optimal performance characteristics for specific applications.
Key Takeaways
-
1
Porous Structure
Electrode porosity affects ion diffusion, electron conduction, and reaction kinetics.
-
2
Manufacturing Impact
Process parameters such as particle size, binder content, and coating thickness influence electrode performance.
-
3
Characterization Techniques
Advanced methods like EIS, SEM, and computational modeling are used to optimize electrode design.
-
4
Performance Trade-offs
Balancing energy density, power density, and cycle life requires careful electrode engineering.
Lithium Ion Battery Manufacturing Process
The manufacturing of lithium ion batteries involves multiple complex steps, each critical to achieving optimal performance and safety.
1 Lithium Ion Battery Pulping
Pulping is the first critical step in electrode manufacturing, where active materials, conductive additives, binders, and solvents are mixed to form a homogeneous slurry.
Key Components
Active materials, conductive carbon, binder, solvent
Mixing Methods
Planetary mixers, high-shear mixers
Proper dispersion and homogenization are critical to ensure uniform electrode properties and performance.Lithium ion battery disposal.
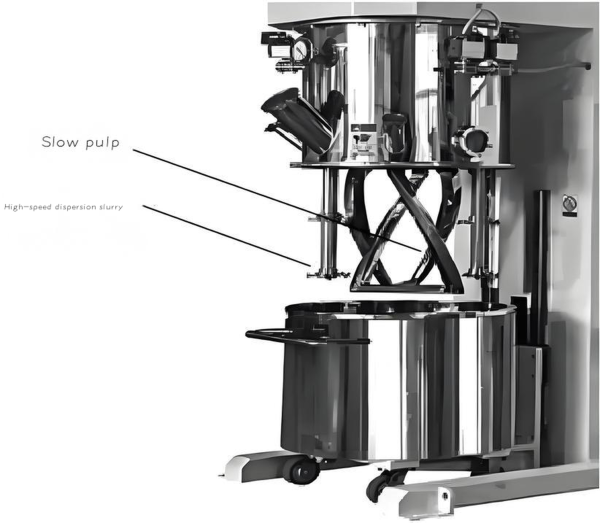

2 Lithium Ion Battery Coating
The slurry is coated onto a current collector foil (aluminum for cathodes, copper for anodes) using techniques such as slot die coating, comma coating, or gravure coating.lithium ion battery.
Coating Thickness
Typically 50-200 μm, depending on application
Drying Process
Removes solvent, critical for film formation
Uniform coating thickness and drying are essential to prevent defects and ensure consistent battery performance.
3 Lithium Ion Battery Electrode Calendaring
Calendaring, or roll pressing, is used to compress the coated electrodes to increase their density, improve electrical contact, and control porosity.lithium ion marine battery.
Key Parameters
Pressure, temperature, roll speed
Density Control
Higher density increases energy density but may reduce power
Optimal calendaring parameters are critical to balance energy density, power performance, and cycle life.


4 Lithium Ion Battery Electrode Cutting
Cut electrodes into the desired size and shape using precision cutting techniques such as die cutting, laser cutting, or rotary cutting—this level of precision is critical to avoid the risk of lithium ion battery explosion (a key concern often referenced in discussions of "lithium ion battery explosive" risks).
Cutting Methods
Die cutting, laser cutting, shear cutting
Edge Quality
Critical to prevent short circuits and improve safety
Precise cutting and clean edges are essential to prevent burrs and debris that could cause internal short circuits.
5 Lithium Ion Battery Assembly
The battery assembly process involves stacking or winding the electrodes with separators between them, followed by insertion into a battery case.
Cell Designs
Cylindrical, prismatic, pouch
Separator Requirements
Electrical insulation, high ionic conductivity
Proper alignment and tension control during stacking or winding are critical to ensure uniform performance and prevent short circuits.


6 Lithium Ion Battery Welding
Welding is used to connect the electrodes to the current collectors and terminals in lithium ion vehicle battery, ensuring low resistance electrical connections.
Welding Techniques
Laser welding, ultrasonic welding, resistance welding
Material Compatibility
Aluminum and copper require different welding parameters
High-quality welds are essential to minimize resistance, reduce heat generation, and ensure long-term reliability.
7 Lithium Ion Battery Formation
Against the backdrop of the lithium-ion batteries checked luggage ban—a measure to address transportation safety risks of lithium-ion batteries—Formation (the initial charge-discharge cycle) becomes even more critical: it activates the battery and forms the solid electrolyte interphase (SEI) on the anode surface, laying a foundation for stable and safe battery performance.
SEI Function
Protects anode, allows lithium ion passage
Formation Parameters
Voltage, current, temperature, time
The quality of the SEI layer significantly impacts battery performance, cycle life, and safety.


8 Power Lithium Ion Batteries
Beyond scenarios of lithium ion battery and flying (such as aerial work drones that rely on high-power batteries), power lithium ion batteries are specifically designed for other high-power applications such as electric vehicles and grid energy storage.
Key Requirements
High power density, long cycle life, safety
Typical Chemistries
NMC, NCA, LFP
Power batteries require specialized design and manufacturing processes to meet the demanding requirements of electric vehicles and other high-power applications.
Advanced Lithium Ion Batteries and Beyond
Lithium-Sulfur Batteries
For applications requiring high energy storage—where lithium polymer li polymer battery vs lithium ion battery may fall short in energy density despite their maturity—lithium-sulfur batteries offer a promising alternative: high theoretical energy density (2600 Wh/kg) and low cost.
- High energy density
- Low-cost sulfur cathode
- Challenges in cycle life and polysulfide shuttling
Solid-State Batteries
Solid-state batteries use solid electrolytes instead of liquid electrolytes, offering improved safety, higher energy density, and longer cycle life.
- Enhanced safety
- Higher energy density
- Challenges in solid electrolyte conductivity
Sustainable Solutions
Research is ongoing to develop more sustainable lithium ion batteries with reduced environmental impact and improved resource efficiency.
- Recycling technologies
- Use of abundant materials
- Greener manufacturing processes
Applications of Lithium Ion Batteries
Lithium ion batteries power a wide range of applications from portable electronics to electric vehicles and grid energy storage.
Consumer Electronics
Lithium ion batteries are the primary power source for smartphones, laptops, tablets, and wearable devices due to their high energy density and long cycle life.
- Smartphones and tablets
- Laptops and notebooks
- Wearable devices

Electric Vehicles
The automotive industry relies heavily on lithium ion batteries to power electric vehicles, hybrid vehicles, and plug-in hybrid electric vehicles.
- Battery electric vehicles (BEVs)
- Hybrid electric vehicles (HEVs)
- Plug-in hybrid electric vehicles (PHEVs)

Energy Storage Systems
Lithium ion batteries play a crucial role in grid energy storage, renewable energy integration, and backup power systems.
- Grid-scale energy storage
- Solar and wind energy integration
- Uninterruptible power supplies (UPS)
Market Trends and Future Outlook
Market Growth
The global lithium ion battery market is expected to reach $129.3 billion by 2027, growing at a CAGR of 18.0% from 2020 to 2027. The increasing demand for electric vehicles and renewable energy storage is the primary driver of this growth.
Future Developments
Future developments in lithium ion battery technology focus on increasing energy density, reducing costs, improving safety, and enhancing sustainability through recycling and the use of more abundant materials.
Higher Energy Density
Development of new cathode and anode materials to increase energy density beyond 300 Wh/kg.
Faster Charging
Research on materials and electrolytes to enable ultra-fast charging capabilities.
Recycling Technologies
Advancements in battery recycling to improve resource efficiency and reduce environmental impact.
Enhanced Safety
Development of safer electrolytes and battery management systems to prevent thermal runaway.
Contact Us
Have questions about lithium ion battery technology or manufacturing processes? Our experts are ready to assist you.